Tuesday, October 11, 2016
Long-term Warfarin use may increase dementia rates in AF patients

Tuesday, January 31, 2017
Apixaban effective in polypharmacy setting
Sunday, August 14, 2011
Study Pits New Blood Thinner Against Warfarin For Irregular Heartbeat
"a reasonable alternative to warfarin, with less intracranial or fatal bleeding." claims the lead researcher, Dr. Manesh R. Patel...
Ref : http://www.newsroom.heart.org/index.php?s=43&item=1191
Monday, January 4, 2010
Dabigatran etexilate a better drug than warfarin for VTE?.
Sunday, November 7, 2010
FDA approves Pradaxa to prevent stroke in people with atrial fibrillation....
Pradaxa is an anticoagulant that acts by inhibiting thrombin, an enzyme in the blood that is involved in blood clotting. The safety and efficacy of Pradaxa were studied in a clinical trial comparing Pradaxa with the anticoagulant warfarin. In the trial, patients taking Pradaxa had fewer strokes than those who took warfarin.
"Unlike warfarin, which requires patients to undergo periodic monitoring with blood tests, such monitoring is not necessary for Pradaxa," Dr. Norman Stockbridge(director of the Division of Cardiovascular and Renal Products in the FDA's ) says.
Pradaxa, manufactured by Boehringer Ingelheim Pharmaceuticals Inc. of Ridgefield, Conn., will be available in 75 milligram and 150 milligram capsules....
Ref : http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm230241.htm
Thursday, July 21, 2016
New oral blood thinners can decrease stroke risk in atrial fibrillation patients without frequent monitoring

 Dabigatran
Dabigatran  Rivaroxaban (BAY 59-7939)
Rivaroxaban (BAY 59-7939) Apixaban
Apixaban  Edoxaban
EdoxabanWednesday, January 11, 2012
Dabigatran, New Blood Thinner Linked To Higher Heart Attack Risk
Monday, May 28, 2012
Flavonoid Compound Found in Foods and Supplements May Prevent the Formation of Blood Clots, Study Suggests...
"Approximately half of all morbidity and mortality in the United States can be attributed to heart attack or stroke."..
Thursday, February 9, 2012
New Anti-Clotting Drug May Cut Brain Bleeding Risk: Study
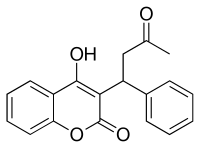
In continuation of my update on rivaroxaban (Xarelto)
In a new study, researchers led by Dr. Graeme Hankey, a neurologist at the Royal Perth Hospital and University of Western Australia, followed more than 14,000 people who took anti-clotting drugs for a median of two years. Of those patients, 136 had bleeding in the brain.
Monday, August 14, 2017
CBD oil may reduce frequency and severity of seizures in patients with epilepsy, UAB study shows

"It is encouraging that both frequency and severity of seizures appear to improve in the majority of patients in our study, patients who have limited treatment options," said Jerzy P. Szaflarski, M.D., Ph.D., professor in the Department of Neurology and director of the UAB Epilepsy Center. "Our research adds to the evidence that CBD may reduce frequency of seizures, but we also found that it appears to decrease the severity of seizures, which is a new finding."
"These are encouraging results, but it is important to note that each patient may respond differently to CBD, and the dose for optimal seizures control varies," said Martina Bebin, M.D., professor of neurology and co-primary investigator of the CBD studies. "There appears to be an optimal CBD dose range where the patient achieves maximum benefit. If outside this CBD dosing range, the seizure frequency may not improve and may even increase. More research is needed, including determining why and how CBD helps some people with epilepsy but not others."



