Thursday, October 20, 2016
Combination of COX-2-selective NSAID with PPI can reduce risk of stomach, intestinal ulcers
Tuesday, September 6, 2016
Sun Pharma Receives FDA Approval For BromSite (bromfenac ophthalmic solution)

Monday, July 2, 2012
Cobiprostone shows promise against NSAID-induced gastric mucosal injury
Tuesday, January 12, 2010
Celecoxib reduces the risk of common skin cancer in humans.....
For the current research, Tang and her colleagues capitalized on a previous finding suggesting that celecoxib, a NSAID, can inhibit the development of a different kind of skin cancer, squamous cell carcinoma, in mice. They wondered if the drug, sold by the pharmaceutical company Pfizer under the brand names Celebrex and Onsenal, would have a similar effect on the more common basal cell carcinoma.
Celecoxib is thought to work to prevent or slow cancer growth by interfering with the action of an enzyme called Cox-2, which causes tissue inflammation (pro inflammator). Celecoxib has both pain-killing (analgesic) and anti-inflammatory properties. Chronic inflammation has long been associated with the development of many types of cancer, and celecoxib has been shown in clinical trials to reduce the incidence of colon cancer in people with a genetic predisposition to the disease.
Interestingly, researchers stopped the clinical trials in 2003 (from 2001) when the study lead to high risk of heart attack and stroke in patients taking a different NSAID. (Rofecoxib, Vioxx by Merck & Co. was withdrawn from the market by Merck in 2004 and Tang's trial was discontinued that year in response to ongoing concerns about long-term treatment with Cox-2 inhibitors). At that time, most participants had received about two years of drug treatment. No patient died or suffered adverse cardiovascular events due to their participation in the trial. Although drug treatment had been discontinued, the researchers continued to monitor basal cell carcinoma formation in people who had received the drug or placebo for an additional year to complete the three-year study. They found that, although both groups continued to develop new cancers during the study, oral celecoxib treatment decreased the growth of skin tumors by about 50 percent as compared to placebo in participants who entered the trial with 15 or fewer basal cell carcinomas. Celecoxib treatment also reduced the overall tumor burden in the group of patients (where in the carcinomas are removed upon diagnosis in most people).
Now the lead researcher Dr. Tang is continuing her focus on skin cancer prevention at Stanford. She's currently investigating whether it's possible to develop a topical formulation of the drug that can be applied directly to the skin to achieve a similar protective effect without associated cardiovascular risk. Hope she will get positive results via topical formulation .....
In my opinion its really a great achievement.We know that compounds with selective inhibitors of 5-LO (Lipoxygenase) and COX (Cyclooxegenase, that too COX-II) will be the best NSAIDs without any ulcerogenecity, its good see that the same compounds can be used to treat skin cancer....
Ref : http://med.stanford.edu/ism/2010/january/tang.html
Friday, June 18, 2010
Sulindac inhibits tumor growth !...
"Depending on the conditions, the same protein, such as RXRα, can either kill cancer cells or promote their growth," Dr. Zhang said. "The addition of K-80003 shifts that balance by blocking survival pathways and sensitizing cancer cells to triggers of apoptosis."
Ref : http://www.cell.com/cancer-cell/retrieve/pii/S1535610810001595
Wednesday, December 3, 2025
FDA Approves Xifyrm (meloxicam) Injection for the Management of Moderate-to-Severe Pain in Adults
Azurity Pharmaceuticals, Inc. announced the U.S. Food and Drug Administration (FDA) approval of Xifyrm (meloxicam injection) an IV non-steroidal anti-inflammatory drug (NSAID) that offers once daily dosing.
Xifyrm is indicated for use in adults for the management of moderate-to-severe pain, alone or in combination with non-NSAID analgesics. Xifyrm provides a non-opioid analgesic in a 30mg/mL vial for IV bolus injection over 15 seconds. Because of delayed onset of analgesia, Xifyrm alone is not recommended for use when rapid onset of analgesia is required.
“Xifyrm demonstrates our commitment to improving patient care by providing an alternate dosage form for pain management,” said Ron Scarboro, CEO at Azurity Pharmaceuticals. “Xifyrm addresses an important clinical need, especially for patients requiring a non-opioid component to multimodal analgesia strategies.”
Xifyrm will be available in the coming weeks. For full prescribing information, including boxed warning and safety profile, please visit www.Xifyrm.com
Wednesday, April 10, 2013
Pain reliever shows anti-viral activity against flu
New influenza vaccines must be developed annually, because the surface proteins they target mutate rapidly, the way cars used to get a whole new look every year. The researchers, led by Anny Slama-Schwok of the Institut National de la Recherche Agronomique, Jouy en Josas, France, found a much more stable, reliable target for anti-influenza activity. The so-called ribonucleoprotein complexes are necessary for replication, and the researchers realized they could target the nucleoprotein, preventing assembly of the complexes. Because of its vital function, the nucleoprotein is highly conserved, making it a good potential target for antiviral drugs.
Sunday, February 24, 2013
Meloxicam recognizes and treats osteoarthritis in cats
Thursday, January 28, 2010
Naproxcinod a better NSAID.....
Tuesday, November 24, 2009
Vardenafil (PDE5 inhibitor) as antiulcer agent?

We know that most of the NSAIDs are associated with ulcerogenecity. Though there are many compounds with different mode of action have been tested (and some of them are being used) to treat the peptic ulcer, compounds with phosphodiesterase 5 inhibitor were not tested before Dr. Karakaya of Zonguldak Karaelmas University-who have reported that Vardenafil can be used to treat the NSAID-induced gastric ulcer. As per the claim by the researchers the activity is dose dependent.
Ref : http://www.wjgnet.com/1007-9327/abstract_en.asp?f=5091&v=15
Friday, May 11, 2012
A new drug to manage resistant chronic pain
Ref : http://www.biolinerx.com/default.asp?pageid=14&itemid=24
A new drug to manage resistant chronic pain
Saturday, September 15, 2012
Tolfenamic acid appears to reduce esophageal tumors
Tolfenamic acid appears to reduce esophageal tumors
Thursday, May 17, 2012
Scientists Spot How Cox-2 Painkillers Raise Heart Risks
"It's really about a rock and a hard place," said Dr. Christopher Cannon, a cardiologist at Brigham and Women's Hospital in Boston. "There's a balance in the bloodstream of clotting and vasoconstriction, as well as protection against clotting and vasodilation, which means that there's a constant balance of clotting and preventing clotting, and constricting arteries and dilating arteries."
"But with cox-2 inhibitors, they have found that you knock the protective side of that balance off," Cannon said. "And then you're left only with the constrictive part, which means the drugs up the risk for clotting and arterial constriction."
"This problem is bigger than just Vioxx, which no longer exists," he added. "It applies to every single NSAID (non-steroidal anti-inflammatory drug), because with all NSAIDs -- including Celebrex and ibuprofen, which zillions of people take -- the same issue exists. You block out the good stuff and leave the bad stuff unchecked. The one exception is Naproxen, which has an anti-platelet effect that seems to work against stroke and heart attack risk."
Tuesday, September 4, 2018
FDA Approves Consensi (amlodipine and celecoxib) for Treatment of Hypertension and Osteoarthritis Pain
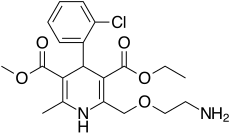
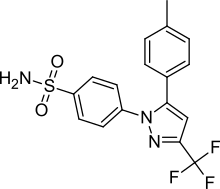
“We are very pleased with Consensi’s approval and would like to thank the members of Kitov’s team, consultants and investigators, as well as the FDA’s Division of Cardiovascular and Renal Products, for all of their support and assistance,” said Dr. J. Paul Waymack, Chairman of Kitov's Board and Chief Medical Officer. “Consensi provides a safe and effective combination treatment option for the millions of Americans who suffer from osteoarthritis pain and hypertension.
“Now that Consensi has been approved for marketing, our clinical and regulatory teams will focus on leveraging their drug development expertise to advance NT219, an exciting investigational new drug candidate currently in development for various oncology indications.”
“Over 50 million Americans suffer from osteoarthritis. About 1 of 3 U.S. adults or about 75 million people have high blood pressure*, known as the “silent killer” due to the absence of noticeable symptoms. As a result, patients’ adherence to the hypertension treatment regimen is low. We believe that Consensi, as a single pill combination treatment for osteoarthritis and hypertension, presents a unique value proposition of potentially increasing treatment adherence.
“We recently expanded our commercialization network for Consensi by securing a second licensing agreement in Asia with a major Chinese pharmaceutical company. The FDA approval of Consensi puts us in a stronger position towards securing commercial partnerships for the U.S. and other key territories.”
Thursday, April 1, 2010
New anti-inflammatory drug shows promise for treating inflammatory disorders
Ref : John L Wallace et. al., British Journal of Pharmacology, 159(6), 1236 - 1246
Wednesday, March 17, 2010
Salsalate may be useful for the treatment of patients with type 2 diabetes .....
Now researchers from Harvard Medical School, lead by Dr. Allison Goldfine, have come up with interesting finding, i.e., Salsalate may be useful for the treatment of patients with type 2 diabetes as well. In a three-month trial of people with type 2 diabetes , those who took the drug showed significantly improved blood glucose levels.
Friday, May 19, 2017
Commonly used anti-inflammatory drug shows potential to treat Alzheimer's disease

"Our research shows for the first time that mefenamic acid, a simple Non-Steroidal Anti Inflammatory Drug can target an important inflammatory pathway called the NLRP3 inflammasome , which damages brain cells."
"However, much more work needs to be done until we can say with certainty that it will tackle the disease in humans as mouse models don't always faithfully replicate the human disease."Because this drug is already available and the toxicity and pharmacokinetics of the drug is known, the time for it to reach patients should, in theory, be shorter than if we were developing completely new drugs.
"These promising lab results identify a class of existing drugs that have potential to treat Alzheimer's disease by blocking a particular part of the immune response. However, these drugs are not without side effects and should not be taken for Alzheimer's disease at this stage - studies in people are needed first."
Monday, May 16, 2016
Steroid May Be Safe, Effective Gout Treatment, Study Finds
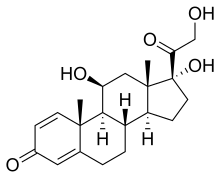 (Prednisole)
(Prednisole)  (Indomethacin)
(Indomethacin)









