In continuation of my update on Tenofovir
Tenofovir disoproxil fumarate (TDF), an antiviral drug commonly prescribed to treat hepatitis B infection, does not significantly reduce mother-to-child transmission of hepatitis B virus when taken during pregnancy and after delivery, according to a phase III clinical trial in Thailand funded by the National Institutes of Health. The study tested TDF therapy in addition to the standard preventative regimen — administration of hepatitis B vaccine and protective antibodies at birth — to explore the drug’s potential effects on mother-to-child transmission rates. The results appear in the New England Journal of Medicine.
“Limited evidence of the benefit of using antiviral drugs to prevent mother-to-child transmission of hepatitis B has led to conflicting practice recommendations around the world,” said Nahida Chakhtoura, M.D., a study team member and medical officer at NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). “Our study suggests that adding TDF to the current regimen seems to have little effect on infant infection rates when transmission rates are already low.”
To prevent infection, WHO recommends that all newborns receive their first dose of hepatitis B vaccine within 24 hours of delivery. Infants born to hepatitis B-infected mothers are also given protective antibodies called hepatitis B immune globulin (HBIG). However, mother-to-child transmission can still occur in women with high levels of virus in their blood, as well as those with mutated versions of the virus.
The current study was conducted at 17 hospitals of the Ministry of Public Health in Thailand. It screened more than 2,500 women for eligibility and enrolled 331 pregnant women with hepatitis B. The women received placebo (163) or TDF (168) at intervals from 28 weeks of pregnancy to two months after delivery. All infants received standard hepatitis B preventatives given in Thailand, which include HBIG at birth and five doses of the hepatitis B vaccine by age 6 months (which differs from the three doses given in the United States). A total of 294 infants (147 in each group) were followed through age 6 months.
Three infants in the placebo group had hepatitis B infection at age 6 months, compared to zero infants in the TDF treatment group. Given the unexpectedly low transmission rate in the placebo group, the researchers concluded that the addition of TDF to current recommendations did not significantly reduce mother-to-child transmission of the virus.
“We observed no treatment-related safety concerns for the mothers or infants and no significant differences in infant growth,” said the study’s lead author Gonzague Jourdain, M.D., Ph.D., of Thailand’s Chiang Mai University, the Harvard T.H. Chan School of Public Health and France’s IRD (Institut de recherche pour le développement). “These safety data also are relevant for pregnant women receiving TDF as part of HIV treatment or HIV pre-exposure prophylaxis.”
According to the study authors, the clinical trial had enough participants to detect statistical differences if the transmission rate in the placebo group reached at least 12 percent, a rate observed in previous studies. Though the reasons are unknown, the researchers speculate that the lower transmission rate seen in the study may relate to the number of doses of hepatitis B vaccine given to infants in Thailand, lower rates of amniocentesis and Cesarean section deliveries in this study, or the lower prevalence of mutated viruses that result in higher vaccine efficacy in Thailand compared to other countries.
Ref : http://www.nejm.org/doi/full/10.1056/NEJMoa1708131

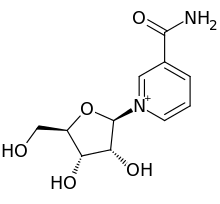

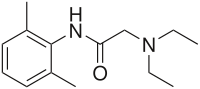
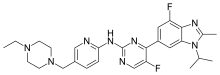
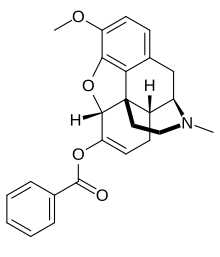 benzhydrocodone
benzhydrocodone