Acucela Inc. a clinical-stage ophthalmology company that specializes in identifying and developing novel therapeutics to treat and slow the progression of sight-threatening ophthalmic diseases, announced today top-line results from the Phase 2b/3 clinical trial (S.E.A.T.T.L.E. study) of the investigational visual cycle modulator emixustat hydrochloride (emixustat).
 emixustat
emixustat
The study enrolled 508 patients with geographic atrophy (GA) secondary to age-related macular degeneration (AMD). The study did not meet its primary endpoint with none of the treatment groups showing a significant difference in lesion growth rate from placebo. The lesion growth rates over 24 months for the 10mg, 5mg, 2.5mg and placebo groups were 1.84 mm2/year, 1.83 mm2/year, 1.69 mm2/year, 1.69 mm2/year, respectively1. There was no significant difference in the mean change of best corrected visual acuity from baseline to month 24 between treatment groups. There was a small numerical treatment difference observed in certain patients with specific genetic profiles in favor of emixustat.
“This is an unfortunate result for patients and physicians who hoped for a treatment for this debilitating disease. We hope to gain important information from this study to better understand this disease and its progression,” said Philip Rosenfeld, MD, Professor of Ophthalmology, Bascom Palmer Eye Institute, University of Miami.
An analysis of the two-year clinical data from the S.E.A.T.T.L.E study showed that adverse events were similar to those seen in earlier trials of emixustat. They include delayed dark adaptation and chromatopsia. There appeared to be no imbalance in serious adverse events between emixustat and the placebo group.
“We are carefully reviewing the data in geographic atrophy before we decide on our next steps with emixustat in this indication. We will continue to advance our in-licensed projects as well as our in-house research," stated Ryo Kubota, MD, PhD, and Chairman, President and CEO of Acucela.
Further analysis of the clinical data from the S.E.A.T.T.L.E. study will be made in collaboration with Otsuka Pharmaceutical in the ensuing months. Acucela has an ongoing pilot study to explore the benefits of emixustat for the treatment of proliferative diabetic retinopathy. Acucela is also considering the initiation of a study to explore the potential benefits of emixustat in Stargardt Disease.
About Emixustat Hydrochloride
Emixustat hydrochloride (emixustat) is an orally administered small molecule that inhibits RPE65, an enzyme crucial to the visual cycle, the chemical pathway in the retina central to the initiation of visual perception. Emixustat is being developed by Acucela in collaboration with Otsuka Pharmaceutical Co., Ltd. (“Otsuka”). Acucela and Otsuka share commercial rights for emixustat in the USA. Otsuka has exclusive rights in Japan, Asia and other countries, while Acucela has exclusive rights in Europe and other countries.
About The Safety and Efficacy Assessment Treatment Trials of Emixustat Hydrochloride (the S.E.A.T.T.L.E.) Study
The S.E.A.T.T.L.E study compared the efficacy and safety of emixustat to placebo for the treatment of geographic atrophy (GA) secondary to dry age-related macular degeneration (AMD). A total of 508 subjects were randomized to receive emixustat 2.5 mg, 5 mg, 10 mg, or placebo, administered orally once daily for up to 24 months. The primary efficacy endpoint was the mean rate of change from baseline in the total area of the GA lesion(s) in the study eye as imaged by fundus autofluorescence. Safety and tolerability were assessed on the basis of ocular and non-ocular adverse events, serious adverse events, ophthalmic examination findings, vital signs, physical examination findings, electrocardiogram findings, and laboratory analyses.
About Geographic Atrophy Secondary to Age-related Macular Degeneration
Geographic atrophy (GA) is a severe and advanced form of age-related macular degeneration (AMD), affecting more than 9 million people worldwide (Market Scope, The Global Retinal Pharmaceuticals & Biologic Market, 2015). In GA, the center of the retina (the macula) responsible for high acuity and color vision becomes atrophic; the atrophic lesion grows over time, eventually leading to irreversible blindness. GA is typically present in both eyes and patients frequently report problems with every day activities such as reading and recognizing faces. GA represents a significant unmet medical need as there are currently no approved treatments for this condition.

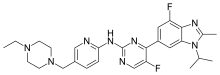
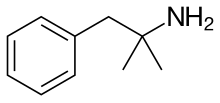 phentermine
phentermine 
 lorcaserin
lorcaserin Bupropion/naltrexone
Bupropion/naltrexone