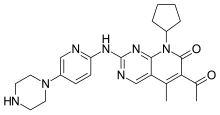
We know that, Belatacept (trade name Nulojix) is a fusion protein composed of the Fc fragment of a human IgG1 immunoglobulin linked to the extracellular domain of CTLA-4, which is a molecule crucial in the regulation of T-cell costimulation, selectively blocking the process of T-cell activation. It is intended to provide extended graft survival while limiting the toxicity generated by standard immune suppressing regimens, such as calcineurin inhibitors. It differs from abatacept (Orencia) by only 2 amino acids.
Belatacept was developed by Bristol-Myers-Squibb and approved by the U.S. Food and Drug Administration on June 15, 2011.
Now A newer drug used for preventing organ rejection might improve the long-term outlook for kidney transplant recipients, a new study finds.
Over seven years, patients given the drug belatacept (brand name: Nulojix) were 43 percent less likely to die or see their donor kidney fail compared to patients given an older drug called cyclosporine.
Experts said the findings should encourage more doctors and patients to choose belatacept over standard anti-rejection medications.
"This is a potentially transformational drug," said study lead researcher Dr. Flavio Vincenti, a transplant specialist at the University of California, San Francisco.
The study -- funded by the drug's maker, Bristol-Myers Squibb -- was published in the Jan. 28 issue of the New England Journal of Medicine.
Belatacept was first approved by the U.S. Food and Drug Administration in 2011 for preventing organ rejection after a kidney transplant. That was based on a three-year trial showing that the drug can prevent rejection in the shorter term, according to background information in the study.
Now the new findings prove what experts had hoped -- that belatacept would be better than cyclosporine in the long run, doctors said.