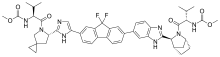
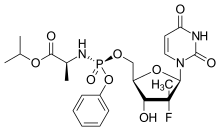
Alios BioPharma, Inc., part of the Janssen Pharmaceutical Companies announced that the New England Journal of Medicine (NEJM) will publish findings from a respiratory syncytial virus (RSV) challenge study for ALS-008176, a cytidine nucleoside analog with activity against RSV. Among infants and young children, RSV is the leading cause of severe respiratory illness and remains the most frequent cause of hospitalization in industrialized countries. This Phase 2a study has now established human proof-of-concept for the antiviral activity of ALS-008176 in healthy adults and highlights its potential as a therapy for managing clinical disease in naturally infected patients.
In this randomized, double-blind study, 62 healthy volunteers were inoculated with RSV and subsequently randomized to receive ALS-008176 or placebo. Compared to placebo, treatment with ALS-00876 resulted in a significant reduction of viral load (73-88% reduction in viral load area under the curve) and faster viral clearance (1.3–2.3 days vs 7.2 days) versus placebo. At the time that the peak viral load occurred in the placebo group, the mean viral load in each of the three ALS-007186 treatment groups was more than one thousand times lower. In addition, statistically significant reductions in symptom scores and a reduction of the amount of congesting respiratory secretions were also observed.