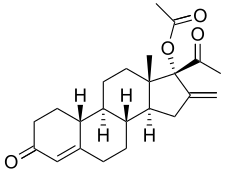
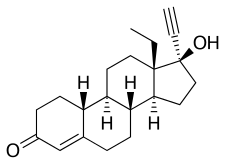
Segesterone acetate
Ethinylestradiol
The Population Council, a global nonprofit research organization, announced it has received U.S. Food and Drug Administration (FDA) approval for Annovera (segesterone acetate and ethinyl estradiol vaginal system), the first and only contraceptive that provides an entire year of protection against unintended pregnancy while fully under a woman's control. The approval marks an important step toward expanding contraceptive options for women.
Annovera is the first in a new class of contraceptives. It is a soft, reusable, flexible silicone ring (2 ¼ inches diameter) that can be inserted and removed by a woman herself. Left in place for 21 days and removed for 7 days each cycle, it is indicated to prevent pregnancy for up to a year and does not require refrigeration, which is particularly important for distribution and use in low-resource settings. Annovera has not been adequately evaluated in women with a body mass index (BMI) greater than 29 kg/m2.
"Nearly half of all pregnancies in the U.S. are unintended, which can increase health risks for mom and baby," said Population Council President, Julia Bunting. "For more than 60 years, the Population Council has been at the vanguard of global efforts to develop innovative family planning methods that meet women's needs. Having a single contraceptive system that provides a full year of protection while under a woman's control could be a game-changer for some women."
According to the Center for Disease Control, more than 43 million women in the U.S. are at risk of unintended pregnancy. Women with unintended pregnancies are less likely to receive proper prenatal care; are more likely to have premature and low-birth-weight infants; and have increased physical and mental health risks. Providing women with a range of contraceptive options that better meet their family planning needs helps reduce unintended pregnancy and improves outcomes.
"I am delighted to have Annovera as a new family planning option for women who want greater choice, convenience and control," said Anita Nelson, M.D., professor and chair, Obstetrics and Gynecology Western University Health Sciences and a principal investigator of the Phase 3 trials. "The Population Council has been a leader in creatively and collectively addressing women's contraceptive needs. It is exciting they are continuing to help empower women with another contraceptive choice."
The FDA approval of Annovera is based in part on data from 17 clinical trials, including two pivotal Phase 3 safety and efficacy trials. The Phase 3 program enrolled a total of 2,308 women across 27 study sites in the United States, Latin America, Europe, and Australia. Women in the trials were between 18 and 40 years of age and were instructed to use the system over 13 menstrual cycles, or one full year. The Primary Endpoint Pearl Index was 2.98. The data show that Annovera is 97.3% effective in preventing pregnancy when used as directed. Annovera offers a similar risk profile to other combined hormonal contraceptives, including a boxed warning related to increased cardiovascular risk when used while smoking.
In formulating Annovera, researchers at the Population Council's Center for Biomedical Research combined a new progestin (segesterone acetate) with a widely used estrogen (ethinyl estradiol) to develop a single product that can inhibit ovulation for an entire year. A sub-set study of women in the Phase 3 clinical trials ranked Annovera highly in terms of convenience, ease of use and comfort. Nearly 9 in 10 women (89%) surveyed were satisfied with it as a method of contraception. Most participants surveyed experienced no change in sexual pleasure or frequency of sexual intercourse.
"This approval is a key first step toward introducing this product globally and better meeting the sexual and reproductive health needs of women, men and young people in the U.S. and around the world," said Jim Sailer, executive director, Center for Biomedical Research at the Population Council. "We are grateful to the dozens of researchers who have worked on this product, the donors who have funded its development, and most of all, to the thousands of women who volunteered to participate in clinical trials and made this all possible."
Important public and private donors from around the world have supported the research and development of Annovera, including the United States Agency for International Development (USAID), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the Bill & Melinda Gates Foundation, the Avis and Clifford Barrus Medical Foundation, the World Health Organization (WHO), and the Population Council.
Recently, the Population Council announced a license agreement with TherapeuticsMD, an innovative healthcare company focused exclusively on women's health, to make Annovera available to women in the U.S. Through the license agreement, TherapeuticsMD will provide significantly reduced pricing to federally designated Title X family planning clinics serving lower-income women. TherapeuticsMD currently estimates Annovera will be commercially available as early as the third quarter of 2019 and commercially launched as early as the fourth quarter of 2019 or first quarter of 2020. Proceeds from the license agreement will be reinvested into the Population Council's continued research and development programs. The Population Council is continuing efforts to make Annovera available worldwide, including in low- and middle-income countries where more than 214 million women have an unmet need for contraception.
Annovera will be the sixth contraceptive technology that Population Council researchers have developed to address family planning needs around the world and brought to market through commercialization agreements. We estimate that more than 170 million women worldwide are currently using highly effective contraceptives developed by the Population Council or based on our technologies. Other Population Council-developed contraceptives include: the copper IUD ParaGard®; intrauterine system Mirena®; contraceptive implants Norplant® and Jadelle®; and Progering®, the contraceptive vaginal ring for breastfeeding women.
Ref : https://en.wikipedia.org/wiki/Segesterone_acetate
https://en.wikipedia.org/wiki/Ethinylestradiol