The study entitled "A phase II neoadjuvant sequential regimen of docetaxel followed by high-dose epirubicin in combination with cyclophosphamide administered concurrently with trastuzumab. The DECT trial" has recently appeared in the Journal of Cell Physiology, an international, per-reviewed journal focused on cancer-related issues. The authors belong to a multidisciplinary Italian-American team with a long and productive history of collaboration with Prof. Antonio Giordano, Director of the Sbarro Institute for Cancer Research of Philadelphia, Temple University Pennsylvania, USA and the Department of Medicine Surgery and Neuroscience at University of Siena.
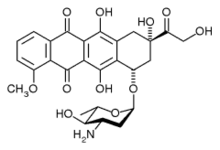 epirubicin
epirubicin
"The use of trastuzumab, a monoclonal antibody targeting the HER2 receptor, has dramatically improved the prognosis of the subgroup of breast cancer patients whose tumors overexpress this specific receptor. One of the greatest challenges in these patients has been combining trastuzumab with extremely effective drugs such as anthracyclines at the cost of an acceptable toxicity. Initial evidence seemed to discourage this approach due to the high-rate of cardiotoxicity, i.e., 27%, reported in the pivotal phase III trial of metastatic breast cancer from Slamon and colleagues. Subsequent studies have partly downsized these results. Yet, several doubts have remained concerning the combined use of these drugs. The DECT trial was design to further address this key question. We also used the data from this randomized trial to interpret treatment efficacy in light of hormonal and metabolic determinants, including the expression of estrogen and progesterone receptors and body mass index," says Prof. Antonio Giordano.
"We enrolled 45 HER2-positive breast cancer patients with locally advanced or operable HER2-positive disease to test the efficacy and toxicity of epirubicin combined with trastuzumab. We observed an exceptionally high rate of responses, particularly in the subgroup of patients with inflammatory disease, and no relevant toxicity, including cardiotoxicity. In addition, some specific disease-and patient-related features were associated with better outcomes. More specifically, the highest chances of optimal response were associated with the lack of hormone receptors and higher BMI," says Dr. Maddalena Barba, researcher at the Regina Elena National Cancer Institute of Rome.
"Although current guidelines discourage from the concurrent use of trastuzumab and anthracyclines in HER2-positive breast cancer, we challenged once more the available evidence by administering a less cardiotoxic anthracycline, i.e., epirubicin at a high dose. The results obtained were remarkable in terms of efficacy and absolutely encouraging in terms of toxicity. In addition, our finding on BMI may deserve further investigation in future studies of HER2-positive breast cancer. The goal we pursue is to define the HER2-positive patient profile which better matches with the highest efficacy at the price of an absolutely acceptable toxicity, including cardiotoxicity," clarifies and concludes Prof. Giordano, a renowned expert in breast cancer.

