In continuation of my update on sacubitril/valsartan
Data from the TRANSITION study presented today at the European Society of Cardiology (ESC) Congress in Munich, Germany has shown that Entresto® (sacubitril/valsartan) can be initiated early and safely in a wide range of heart failure patients with reduced ejection fraction (HFrEF) who have been stabilized after hospitalization due to an acute heart failure episode. Patients involved in the study included those with no prior experience of Entresto or conventional HF therapies, as well as those with prior experience of conventional HF therapies.
About half of all heart failure patients have reduced ejection fraction, and optimizing treatment for these patients according to guidelines is critical to reduce the likelihood of another acute episode or dying. However, there is often hesitancy to initiate a new treatment after a hospitalization as these patients are considered 'vulnerable' and unable to tolerate changes in their medication.
"In the weeks following an episode of acute heart failure, patients are very vulnerable and face a high risk of re-hospitalization and death," said Prof. Rolf Wachter, University Hospital Leipzig, Germany and study investigator. "The PARADIGM-HF study showed that sacubitril/valsartan reduces heart failure-related hospitalizations, re-hospitalization and death. TRANSITION shows that sacubitril/valsartan can be initiated early and safely in patients shortly after an acute heart failure episode, providing physicians with added confidence to optimize their care with innovative medicines in heart failure treatment."
In TRANSITION, the safety and tolerability of Entresto were assessed in HFrEF patients after they have been stabilized following an acute heart failure episode. Patients were randomized to initiate Entresto therapy either in the hospital (pre-discharge) or shortly after leaving the hospital (post-discharge). At 10 weeks, more than 86% of patients were receiving Entresto for 2 weeks or longer without interruption and about half of patients in the study achieved the primary endpoint which was a target dose of 200 mg of Entresto twice daily within 10 weeks in both groups. The number of patients who met the primary and secondary endpoints was similar across both treatment arms. The incidence of adverse events and discontinuations of Entresto due to adverse events was also similar in both the in-hospital and the out-patient setting.
"We are encouraged by the findings of TRANSITION which show that Entresto, the new standard of care in heart failure, can be safely initiated in recently hospitalized patients," said Shreeram Aradhye, MD, Chief Medical Officer and Global Head, Medical Affairs, Novartis Pharmaceuticals. "Heart failure is a serious progressive disease with 83% of patients hospitalized at least once for an acute heart failure episode during the course of their condition. Hospitalization provides an opportunity for physicians to optimize heart failure treatment according to guidelines to reduce the likelihood of hospital readmission and death, reduce the burden of hospitalizations, and improve patient outcomes."
Ref : https://www.novartis.com/news/media-releases/novartis-announces-new-data-show-entresto-sacubitrilvalsartan-can-be-initiated-early-safely-hospitalized-patients-after-acute-heart-failure-episode

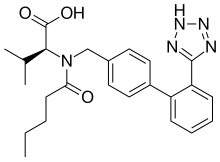
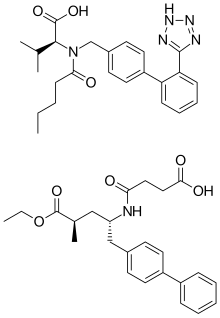
 Sacubitril
Sacubitril
