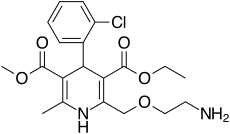
Tuesday, December 2, 2025
FDA Approves Widaplik (telmisartan, amlodipine and indapamide) for the Treatment of Hypertension
Wednesday, March 2, 2022
FDA Approves Norliqva (amlodipine) Oral Solution for Hypertension and Coronary Artery Disease
In continuation of my update on amlodipine besylate
The U.S. Food and Drug Administration has approved Norliqva (amlodipine) oral solution for the treatment of:
- Hypertension in adults and children 6 years and older, to lower blood pressure.
- Coronary artery disease [Chronic Stable Angina, Vasospastic Angina (Prinzmetal’s or Variant Angina) and Angiographically Documented Coronary Artery Disease in patients without heart failure or an ejection fraction <40%.]
Amlodipine is a widely used long-acting calcium channel blocker first approved by the FDA thirty years ago. It is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure, and is thought to reduce the total peripheral resistance and inhibit coronary spasm to relieve angina.
Amlodipine is FDA approved under the brand names Norvasc (amlodipine oral tablets) and Katerzia (amlodipine oral suspension), and the oral tablets are also available as generics.
Norliqva is supplied as a peppermint flavored oral solution containing 1 mg/mL amlodipine as the besylate salt. It is administered once daily.
Most common adverse reactions to amlodipine include edema, dizziness, flushing and palpitation which occurred in a dose related manner. Other adverse reactions not clearly dose-related but reported with an incidence >1.0% are fatigue and nausea.
Tuesday, February 18, 2020
FDA Approves Conjupri (levamlodipine maleate) for the Treatment of Hypertension
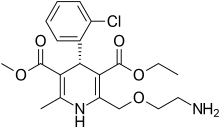
Thursday, January 16, 2020
FDA Approves Katerzia (amlodipine) Oral Suspension for Pediatric Patients 6 Years of Age and Older
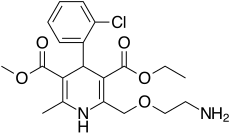
https://en.wikipedia.org/wiki/Amlodipine
Tuesday, September 4, 2018
FDA Approves Consensi (amlodipine and celecoxib) for Treatment of Hypertension and Osteoarthritis Pain

“We are very pleased with Consensi’s approval and would like to thank the members of Kitov’s team, consultants and investigators, as well as the FDA’s Division of Cardiovascular and Renal Products, for all of their support and assistance,” said Dr. J. Paul Waymack, Chairman of Kitov's Board and Chief Medical Officer. “Consensi provides a safe and effective combination treatment option for the millions of Americans who suffer from osteoarthritis pain and hypertension.
“Now that Consensi has been approved for marketing, our clinical and regulatory teams will focus on leveraging their drug development expertise to advance NT219, an exciting investigational new drug candidate currently in development for various oncology indications.”
“Over 50 million Americans suffer from osteoarthritis. About 1 of 3 U.S. adults or about 75 million people have high blood pressure*, known as the “silent killer” due to the absence of noticeable symptoms. As a result, patients’ adherence to the hypertension treatment regimen is low. We believe that Consensi, as a single pill combination treatment for osteoarthritis and hypertension, presents a unique value proposition of potentially increasing treatment adherence.
“We recently expanded our commercialization network for Consensi by securing a second licensing agreement in Asia with a major Chinese pharmaceutical company. The FDA approval of Consensi puts us in a stronger position towards securing commercial partnerships for the U.S. and other key territories.”
Wednesday, March 23, 2016
Osteoarthritis: pivotal Phase III trial successfully meets primary efficacy endpoint - Medical News Today


Wednesday, November 25, 2009
Nicardipine Hydrochloride injection is back !
Nicardipine is a dihydropyridine calcium-channel blocking agent used for the treatment of vascular disorders such as chronic stable angina, hypertension, and Raynaud's phenomenon. It is available in oral and intravenous formulations. Its mechanism of action and clinical effects closely resemble those of nifedipine and the other dihydropyridines (amlodipine, felodipine), except that nicardipine is more selective for cerebral and coronary blood vessels. Furthermore, nicardipine does not intrinsically decrease myocardial contractility and may be useful in the management of congestive heart failure. Nicardipine also has a longer half-life than nifedipine. Nicardipine was approved by the FDA in December 1988. The patent for both Cardene and Cardene SR expired in October 1995.
Recently, Caraco Pharmaceutical Laboratories, Ltd. has launched Nicardipine Hydrochloride Injection immediately following Sun Pharma's final approval from the US Food and Drug Administration (FDA).
Nicardipine Hydrochloride Injection is indicated for the short-term treatment of hypertension when oral therapy is not feasible or not desirable. These Nicardipine Hydrochloride Injections are available as 25 mg/10ml single use ampuls containing 2.5 mg/ml of the drug....
Source : http://phx.corporate-ir.net/phoenix.zhtml?c=98920&p=irol-newsArticle_Print&ID=1356898&highlight=