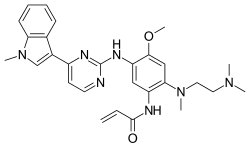
In continuation of my update on lenvatinib
Woodcliff Lake, NJ and Kenilworth, announced today that the U.S. Food and Drug Administration (FDA) approved the kinase inhibitor Lenvima (lenvatinib) for the first-line treatment of patients with unresectable hepatocellular carcinoma (HCC). This approval was based on results from REFLECT (Study 304), where Lenvima demonstrated a proven treatment effect on overall survival (OS) by statistical confirmation of non-inferiority, as well as statistically significant superiority and clinically meaningful improvements in progression-free survival (PFS) and objective response rate (ORR) when compared with sorafenib in patients with previously untreated unresectable HCC.
“Unresectable hepatocellular carcinoma is an extremely difficult-to-treat cancer, with no new first-line systemic therapy options for more than a decade,” said Dr. Ghassan Abou-Alfa, medical oncologist, Memorial Sloan Kettering Cancer Center. “REFLECT is the first-ever positive Phase 3 trial against an active comparator in unresectable HCC. The efficacy and safety data from REFLECT are important findings for oncologists and others in the multidisciplinary teams who treat liver cancer, as well as for our patients who are affected by it.”
Adverse reactions, some of which can be serious or fatal, may occur with Lenvima, including hypertension, cardiac dysfunction, arterial thromboembolic events, hepatotoxicity, renal failure or impairment, proteinuria, diarrhea, fistula formation and gastrointestinal perforation, QT interval prolongation, hypocalcemia, reversible posterior leukoencephalopathy syndrome, hemorrhagic events, impairment of thyroid stimulating hormone suppression/thyroid dysfunction, and wound healing complications. Based on the severity of the adverse reaction, Lenvima should be monitored, withheld or discontinued. Based on its mechanism of action and data from animal reproduction studies, Lenvima can cause fetal harm when administered to a pregnant woman. Females of reproductive potential should be advised to use effective contraception. For more information, see “Important Safety Information” below.
REFLECT showed that Lenvima achieved the primary endpoint, demonstrating a treatment effect on OS by statistical confirmation of non-inferiority to sorafenib. Patients treated with Lenvima experienced a median OS of 13.6 months compared to 12.3 months with sorafenib (HR: 0.92; 95% CI: 0.79–1.06). The OS analysis was conducted when 351 events had occurred in the Lenvima arm and 350 events had occurred in the sorafenib arm, as prespecified in the statistical analysis plan. In addition, Lenvima showed statistically significant superiority and clinically meaningful improvements in the secondary efficacy endpoints of PFS and ORR, as confirmed by a blinded independent imaging review (IIR):
- Median PFS was doubled with Lenvima compared to sorafenib: 7.3 months versus 3.6 months (HR: 0.64; 95% CI: 0.55–0.75; p<0.001) per blinded independent imaging review based on mRECIST criteria, and 7.3 months with Lenvima versus 3.6 months with sorafenib (HR: 0.65; 95% CI: 0.56–0.77) per RECIST 1.1.
- Lenvima showed nearly 3.5 times the ORR of sorafenib: 41% (95% CI: 36-45%) vs. 12% (95% CI: 10-16%) per blinded independent imaging review based on mRECIST criteria, respectively (p<0.001), and 19% (95% CI: 15-22%) with Lenvima versus 7% (95% CI: 4-9%) with sorafenib per RECIST 1.1.
- Per mRECIST: Treatment with Lenvima resulted in complete response (CR) = 2.1% (n=10) vs. 0.8% (n=4) with sorafenib; treatment with Lenvima resulted in partial response (PR) = 38.5% (n=184) vs. 11.6% (n=55) with sorafenib
- Per RECIST 1.1: Treatment with Lenvima resulted in CR = 0.4% (n=2) vs. 0.2% (n=1) with sorafenib; treatment with Lenvima resulted in PR = 18.4% (n=88) vs. 6.3% (n=30) with sorafenib
In addition, median time to progression (TTP) was doubled with Lenvima compared to sorafenib: 7.4 months versus 3.7 months (HR: 0.60; 95% CI: 0.51–0.71; p<0.0001) per blinded independent imaging review based on mRECIST criteria, and 7.4 months with Lenvima versus 3.7 months with sorafenib (HR: 0.61; 95% CI: 0.51–0.72; p<0.0001) per RECIST 1.1. Time to progression is defined as time from randomization to radiological progression. Deaths during follow-up without evidence of radiological progression are censored. This differs from PFS and is less correlative to overall survival.
In REFLECT, the most common adverse reactions (≥20%) observed in patients treated with Lenvima were hypertension, fatigue, diarrhea, decreased appetite, arthralgia/myalgia, decreased weight, abdominal pain, palmar-plantar erythrodysesthesia syndrome, proteinuria, dysphonia, hemorrhagic events, hypothyroidism and nausea. The most common serious adverse reactions (≥2%) reported in patients treated with Lenvima were hepatic encephalopathy (5%), hepatic failure (3%), ascites (3%) and decreased appetite (2%).
The most common adverse reactions (≥20%) observed in patients who received sorafenib were palmar-plantar erythrodysesthesia syndrome, diarrhea, fatigue, hypertension, abdominal pain, decreased appetite, rash, decreased weight and arthralgia/myalgia. The most common serious adverse reactions (≥2%) reported in patients who received sorafenib were ascites (2%) and abdominal pain (2%).
It is also important to note that the dose for Lenvima for patients with unresectable HCC is based on the patient’s weight (12 mg for patients weighing 60 kilograms or more, 8 mg for patients weighing less than 60 kilograms); the recommended dosage and dose adjustments are described in the full prescribing information.
“Eisai strives to be a leading global R&D-based pharmaceutical company, driven by our human health care (hhc) mission to improve the lives of patients and their loved ones,” said Shaji Procida, President and Chief Operating Officer, Eisai Inc., and Commercial Head of the Oncology Business Group, Americas at Eisai. “That purpose is what has propelled us toward this win for patients with unresectable hepatocellular carcinoma. Our goal is to bring monumental solutions to patients and health care providers, changing expectations for the oncology landscape, and we look forward to continuing this work in our ongoing collaboration with Merck.”
“We are pleased by the FDA approval of Lenvima as it marks an important advancement in the treatment of unresectable hepatocellular carcinoma,” said Dr. Roy Baynes, Senior Vice President and Head of Global Clinical Development, Chief Medical Officer, Merck Research Laboratories. “With our shared mission to find solutions for difficult-to-treat cancers, we look forward to working with Eisai to help bring this needed option to patients and physicians.”
Lenvima, a kinase inhibitor, was first approved in the U.S. in February 2015 for patients with locally recurrent or metastatic, progressive, radioactive iodine-refractory differentiated thyroid cancer (DTC). In May 2016, Lenvima was approved in the U.S. in combination with everolimus, for patients with advanced renal cell carcinoma (RCC) following one prior anti-angiogenic therapy. Under the collaboration, Eisai and Merck initiated co-commercialization activities for Lenvima in the U.S. in June 2018. Since the initial launch, more than 10,000 patients were treated with Lenvima, which is approved in more than 50 countries worldwide.
------------------------------------------------------------------------------------------------------------------------
FDA Approves Lenvima (lenvatinib) for First-line Treatment of Unresectable Hepatocellular Carcinoma (HCC)