Wednesday, January 28, 2026
FDA Approves Contepo (fosfomycin) for Injection for the Treatment of Complicated Urinary Tract Infections
Tuesday, September 10, 2024
FDA Approves Exblifep (cefepime/enmetazobactam) for the Treatment of Complicated Urinary Tract Infections,
Allecra Therapeutics (“Allecra”), a biopharmaceutical company developing novel therapies to combat antibiotic resistance, announced today that the U.S. Food and Drug Administration (FDA) has approved Exblifep (cefepime/enmetazobactam), as a treatment for complicated urinary tract infections (cUTIs), including pyelonephritis, in patients 18 years and older. Allecra has also received a five-year marketing exclusivity extension from the FDA as part of the Generating Antibiotic Incentives Now Act (GAIN Act). The GAIN Act, enacted by the U.S. Congress, incentivizes the creation of new anti-infective therapeutics by providing benefits to manufacturers of Qualified Infectious Disease. Products (QIDPs).
"Receiving FDA approval is a tremendous achievement for Allecra and a testament to the hard work and dedication of a small, yet highly focused team of individuals. I extend my sincere congratulations to my colleagues Omar Lahlou and Patrick Velicitat for their leadership and oversight throughout this whole process,” said Iain Buchanan, Supervisory Board Member of Allecra Therapeutics. “As we continue our discussions with strategic partners for product launch in the U.S., we value the FDA’s positive decision on Exblifep’s ability to address a critical unmet medical need for patients.”
The FDA’s approval of Exblifep was supported by a totality of clinical data that demonstrated Exblifep effectiveness against antimicrobial resistance in gram-negative bacteria, especially resistance mediated by both ESBL (Extended Spectrum Beta Lactamases) and AmpC. This included results from Allecra’s Phase 3 ALLIUM trial, which met criteria for non-inferiority and superiority compared to piperacillin/tazobactam in the primary composite outcome of clinical cure and microbiological eradication in patients with cUTIs.
Allecra is the sole holder of a significant patent estate covering Exblifep in major territories with the GAIN Act extending Allecra’s market exclusivity until 2032. Enmetazobactam was first discovered by Orchid Pharma and all rights outside India were assigned to Allecra Therapeutics in 2013. The company has since taken the sole responsibility for the international clinical and regulatory development of Exblifep. Allecra was founded through a strategic partnership formed by Nicholas Benedict, Stuart Shapiro and Edward Currie in conjunction with Orchid Chemicals and Pharmaceuticals Ltd. and Allecra lead investors, Andera Partners, Forbion and EMBL Ventures. The company has concluded exclusive license agreements for Exblifep with Shanghai Haini Pharmaceutical in Greater China and ADVANZ PHARMA in Europe.
Thursday, December 30, 2021
Spero Therapeutics Submits New Drug Application for Tebipenem HBr for the Treatment of Complicated Urinary Tract Infections including Pyelonephritis
Spero Therapeutics, Inc. announced the submission of a new drug application (NDA) to the U.S. Food and Drug Administration (FDA), seeking approval for tebipenem HBr tablets for the treatment of complicated urinary tract infections (cUTI), including pyelonephritis, caused by susceptible microorganisms. If approved, tebipenem HBr would be the only oral carbapenem antibiotic available for use in cUTI.
“With the submission of this NDA, we have taken a major step towards potentially providing a substantial number of appropriate cUTI patients with an oral treatment option that could replace historical use of intravenous (IV) therapy,” said Ankit Mahadevia, M.D., Chief Executive Officer of Spero Therapeutics. “If approved, we believe tebipenem HBr could help patients significantly, and the avoidance of IV administration could lead to reduced healthcare resource utilization. We look forward to working with the FDA during the NDA review process as we prepare for tebipenem HBr’s anticipated launch in the second half of 2022.”
The NDA submission includes previously communicated positive data from the Phase 3 ADAPT-PO trial. This data showed that ADAPT-PO met its primary endpoint by demonstrating that oral tebipenem HBr was statistically non-inferior to IV ertapenem in the treatment of patients with cUTI and patients with acute pyelonephritis (AP).
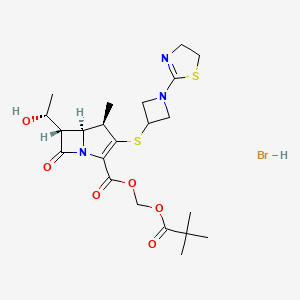
https://pubchem.ncbi.nlm.nih.gov/compound/Tebipenem-pivoxil-hydrobromide
Tuesday, March 3, 2020
FDA Approves Fetroja (cefiderocol) for the Treatment of Complicated Urinary Tract Infections

“Today’s approval provides an additional treatment option for patients with cUTIs who have limited or no alternative treatment options,” said John Farley, M.D., M.P.H., acting director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research. “A key global challenge the FDA faces as a public health agency is addressing the threat of antimicrobial-resistant infections, like cUTIs. This approval represents another step forward in the FDA’s overall efforts to ensure safe and effective antimicrobial drugs are available to patients for treating infections.”


