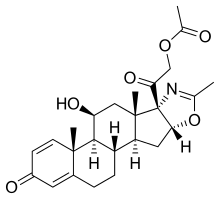
PTC Therapeutics, Inc. (NASDAQ: PTCT) announced that the U.S. Food and Drug Administration (FDA) approved the company's supplemental New Drug Application (sNDA) for Emflaza (deflazacort) to expand its labeling to include patients with Duchenne muscular dystrophy who are between 2- and 5-years-old. Duchenne is a rare childhood genetic disorder that causes progressive irreversible muscle deterioration and weakness. Emflaza was first approved by the FDA in February 2017 for the treatment of Duchenne in patients 5-years and older.
"We are excited to be able to bring Emflaza to younger boys living with Duchenne muscular dystrophy," said Stuart Peltz, Ph.D., Chief Executive Officer of PTC Therapeutics. "The standard of care is to start Emflaza at the time of diagnosis. We believe that treating patients as young as possible, when they still have a substantial amount of muscle, will have the greatest benefit for patients that are two years and older."
https://musculardystrophynews.com/emflaza-deflazacort-duchenne-muscular-dystrophy/
https://en.wikipedia.org/wiki/Deflazacort
