Bottom Line: The investigational anticancer therapeutic abemaciclib, which targets CDK4 and CDK6, showed durable clinical activity when given as continuous single-agent therapy to patients with a variety of cancer types, including breast cancer, non-small cell lung cancer (NSCLC), glioblastoma, and melanoma, according to results from a phase I clinical trial.
Journal in Which the Study was Published: Cancer Discovery, a journal of the American Association for Cancer Research.
Senior authors: Amita Patnaik, MD, associate director of clinical research at South Texas Accelerated Research Therapeutics in San Antonio, Texas, and Geoffrey I. Shapiro, MD, PhD, director of the Early Drug Development Center at the Dana-Farber Cancer Institute in Boston.
Background: In February 2015, the U.S. Food and Drug Administration (FDA) approved the CDK4/6 inhibitor palbociclib (Ibrance) for use in combination with the aromatase inhibitor letrozole for treating postmenopausal women with estrogen receptor-positive, HER2-negative advanced breast cancer.
 letrozole
letrozole 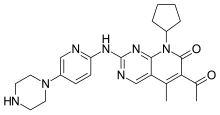 palbociclib
palbociclib
The oral CDK4/6 inhibitor abemaciclib is a very different molecule from palbociclib, with distinct attributes that contribute to its discrete therapeutic effects, in particular, its single-agent activity, according to Shapiro. For example, abemaciclib has greater selectivity for CDK4 compared with palbociclib, which may explain why it does not affect white blood cell counts as severely, allowing it to be taken on a continuous schedule without treatment holidays, he said. Abemaciclib also penetrates the central nervous system, whereas palbociclib does not, raising the possibility that it could be used to treat primary or metastatic brain tumors, he added.
How the Study Was Conducted and Results: Patnaik, Shapiro, and colleagues enrolled 225 patients with a variety of types of advanced cancer in the phase I clinical trial designed to evaluate the safety and preliminary efficacy of abemaciclib. In the dose escalation phase, the researchers determined that the maximum tolerated dose was 200 milligrams (mg) every 12 hours; the dose-limiting toxicity was grade 3 fatigue.
In the expansion phase, single-agent abemaciclib was administered to 47 patients with breast cancer, 68 with NSCLC, 17 with glioblastoma, 26 with melanoma, and 15 with colorectal cancer. Among these patients, the most common treatment-related adverse events were fatigue, diarrhea, nausea, vomiting, anorexia, weight loss, kidney dysfunction, and decreased red and white blood cell counts.
Radiographic responses were observed for some patients with breast cancer, NSCLC, and melanoma. Among the 36 patients with hormone receptor-positive breast cancer, 11 had a partial response, with four of the 11 responders having continued prior endocrine therapy, and an additional 18 patients had stable disease. Among the 68 patients with NSCLC, two had a partial response and 31 had stable disease; one patient who had a partial response and 12 who had stable disease were known to have KRAS-mutant NSCLC. Among the 26 patients with melanoma, one had a partial response and six had stable disease. Three of the 17 patients with glioblastoma had stable disease, with two of them continuing to receive treatment without disease progression for 19 and 23 cycles, respectively.
Author Comment: "These data show that abemaciclib is an oral drug that can be taken on a continuous schedule and achieve durable clinical activity against multiple tumors including breast and lung cancers," said Shapiro.
"The results of the trial supported the FDA decision to grant breakthrough therapy designation to abemaciclib (previously known as LY2835219) for patients with refractory hormone receptor-positive advanced or metastatic breast cancer," added Patnaik.
Limitations: Patnaik explained that because this study included 225 patients with different types of cancer, confirmatory clinical trials in specific patient populations are necessary to precisely define the role of abemaciclib in cancer care. Multiple clinical trials have already been initiated to evaluate abemaciclib as a treatment for certain groups of patients with breast cancer and NSCLC, as well as children with primary brain tumors and adults with brain metastases, she noted.
Investigational drug abemaciclib shows durable clinical activity for variety of cancer types: The investigational anticancer therapeutic abemaciclib, which targets CDK4 and CDK6, showed durable clinical activity when given as continuous single-agent therapy to patients with a variety of cancer types, including breast cancer, non-small cell lung cancer (NSCLC), glioblastoma, and melanoma, according to results from a phase I clinical trial.
