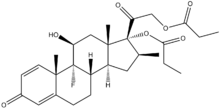
We know that Betamethasone dipropionate (see structure) is a glucocorticoid steroid with anti-inflammatory and immunosuppressive abilities. It is applied as a topical cream, ointment, lotion or gel (Diprolene) to treat itching and other minor skin conditions such as eczema.
Brand names include Alphatrex, Beta-Val, Diprolene, Diprolene AF, Diprosone, and Luxiq.
Minor side effects include dry skin and mild, temporary stinging when applied.
Betamethasone dipropionate is a "super high potency" corticosteroid used to treat inflammatory skin conditions such as dermatitis, eczema andpsoriasis. It is a synthetic analog of the adrenal corticosteroids. Although its exact mechanism of action is not known, it is effective when applied topically to cortico-responsive inflammatory dermatoses...
Dr. Reddy’s announced today that its US subsidiary, Promius Pharma, LLC, U.S. (BSE: 500124, NSE: DRREDDY, NYSE: RDY), has received approval for Sernivo (betamethasone dipropionate) Spray, 0.05% from the U.S. Food and Drug Administration (FDA). Sernivo Spray, a prescription topical steroid, is indicated for the treatment of mild to moderate plaque psoriasis in patients 18 years of age or older. The commercial launch of the product is planned for the coming quarter.
