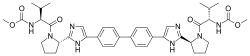
Daclatasvir formerly BMS-790052, trade name Daklinza) is a drug for the treatment of hepatitis C (HCV). It was developed by Bristol-Myers Squibb and was approved in Europe on 22 August 2014. Daklinza gained its FDA approval on July 24, 2015 in the United States; it is approved for Hepatitis C genotype 3 infections.
A generic version of daclatasvir is expected to be approved in India before the end of 2015.
Daclatasvir inhibits the HCV nonstructural protein NS5A. Recent research suggests that it targets two steps of the viral replication process, enabling rapid decline of HCV RNA. Daclatasvir has been tested in combination regimens with pegylated interferon and ribavirin, as well as with other direct-acting antiviral agents including asunaprevir and sofosbuvir....
Now....
EC approves expanded use of Daklinza (daclatasvir) for patients with chronic HCV and HIV co-infection: Bristol-Myers Squibb today announced that the European Commission has approved the expanded use of Daklinza, a first-in-class oral, once-a-day pill used in combination with other treatments as an option for adult patients with chronic hepatitis C virus infection who are co-infected with HIV or who have had a prior liver transplant.
