It is known that Alzheimer’s disease is caused by the formation of amyloid plaques and tau tangles in the brain. A novel compound shows promise in preventing amyloid formation, fighting Alzheimer’s disease development.
Alzheimer’s disease (AD) is the most common form of dementia, affecting about 50 million people worldwide. In the United States, 5.5 million people are living with neurodegenerative diseases, making it the 6th leading cause of death in the country.
AD is caused by the abnormal build-up of proteins, amyloid, and tau, in and around the brain. These proteins form plaques and tangles in brain cells, leading to memory loss and other symptoms. Abnormal proteins form toxic clumps, dubbed as fibrils, inside the brain, affecting brain regions that are vital for brain processes.
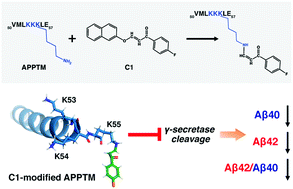
In the study published in the journal Chemical Communications by the Royal Society of Chemistry, reveals that the new compound, known as “C1”, can prevent the enzyme gamma-secretase from producing amyloids.
What is the role of C1?
Amyloid fibrils are made of peptide amyloid-beta, produced when certain enzymes make cuts to the amyloid precursor protein, which is found in the brain cell membrane. A type of covalent gamma-secretase inhibitor, the compound works by blocking the active site of the enzyme, hence, preventing the formation of amyloid.
The team of researchers at the Center for Biotechnology and Interdisciplinary Studies (CBIS) at Rensselaer Polytechnic Institute noted that there were samples of gamma-secretase inhibitors in the past, but these failed since they have severe side effects. What happened is, the inhibitors used stopped all the functions of gamma-secretase.
“Our compound binds to the cleavage site of the precursor protein instead of the enzyme itself, which may avoid many problems associated with traditional enzyme inhibitors,” Chunyu Wang, a professor of biological sciences and author of the study, said.
The team started to screen drugs to determine a potential compound that can target the amyloid precursor protein substrate, blocking the gamma-secretase activity that is tied to amyloid production. Using a computer model, they tested millions of compounds in the hopes of finding the one that can show promise in battling Alzheimer’s disease.
Though there were several candidates found, C1 showed high accuracy and effectiveness in cell cultures and test tubes. The patent for the compound is still pending but the researchers hope that the new drug can be studied more to determine its efficacy in people at a high risk of developing Alzheimer’s disease.
Implications of the compound
The discovery of the novel compound can pave the way for the development of new drugs that can prevent and treat Alzheimer’s disease. The new approach targets the disease, based on tis principal pathology.
Currently, there is no cure for Alzheimer’s disease and the treatment revolves around providing a safe environment for patients. Therapy is also effective in providing support, but scientists are racing to finally find a medicine for the condition.
What is Alzheimer’s disease?
Alzheimer’s disease is a type of dementia, which is a neurodegenerative disease. The disease is irreversible and progressive, which means that it worsens over time. The condition affects brain sections responsible for memory, thinking skills, and cognitive ability. In time, the symptoms worsen, often causing the inability to carry out the simplest tasks or activities of daily living.
The disease first appears in people who are in their mid-60s but can emerge earlier in some cases. Scientists don’t fully understand the exact cause of Alzheimer’s disease, but it may be a combination of various factors. These include age, since older adults are mostly affected, and hereditary because it can run in families. There is also evidences that changes in the brain starting even years before the start of the symptoms may have occurred.
https://pubs.rsc.org/en/Content/ArticleLanding/2020/CC/C9CC09170J#!divAbstract



 Relebactam
Relebactam