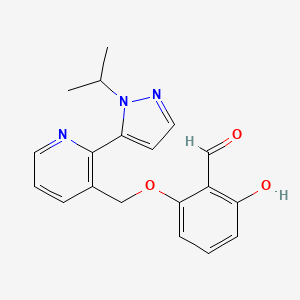
In continuation of my update on Gadavist (gadobutrol)
Bayer announced today the U.S. Food and Drug Administration (FDA) has approved Gadavist (gadobutrol) injection for use in cardiac magnetic resonance (MR) imaging to assess myocardial perfusion (stress, rest) and late gadolinium enhancement in adult patients with known or suspected coronary artery disease (CAD). Gadavist is now the first and only contrast agent FDA approved for use in cardiac MR – an important diagnostic tool for patients with CAD.
"Gadobutrol-enhanced cardiac MR demonstrated efficacy in a large global multicenter clinical trial," said Daniel S. Berman, MD, FACC, Chief of Cardiac Imaging and Nuclear Cardiology at the Cedars-Sinai Heart Institute and the S. Mark Taper Foundation Imaging Center. "The FDA approval is a landmark for making this validated, non-invasive method available to healthcare professionals to evaluate their patients for the most common form of heart disease in the world."
The approval was based on two multinational, non-randomized, blinded-read Phase 3 studies of almost 1,000 adults with suspected or known CAD based on signs and symptoms. Nearly 800 of those patients were evaluated for efficacy. First approved in 2011, cardiac MR is now the fourth FDA approved indication for Gadavist.2
The Society for Cardiovascular Magnetic Resonance recognizes cardiac MR as a non-invasive tool that provides relevant and actionable information to healthcare professionals.3
"We now have an approved contrast agent for use in cardiac MR to assess perfusion and late gadolinium enhancement in less than one hour," said Scott Flamm, MD, MBA, Head of Cardiovascular Imaging, Cleveland Clinic. "A Gadavist-enhanced cardiac MR is a key diagnostic tool, providing additional important clinical information, which can help physicians manage their patients with known or suspected CAD."
A disease that affects approximately 16.5 million Americans, CAD develops when the major blood vessels that supply the heart with blood, oxygen and nutrients (coronary arteries) become damaged or diseased.1,5 Cholesterol-containing deposits (plaque) in the arteries and inflammation are usually the cause of CAD. When plaque builds up, it narrows the coronary arteries, decreasing blood flow to the heart. Eventually, the decreased blood flow may cause chest pain (angina), shortness of breath, or other coronary artery disease signs and symptoms. A complete blockage can cause a heart attack.4
"This latest FDA approval represents another first from Bayer, as Gadavist is the first and only contrast agent approved for cardiac MR," said Dennis Durmis, SVP and Head of Americas Region at Bayer Radiology. "Not only does this approval add to our existing indications for Gadavist, expanding scientific knowledge, but also underscores our dedication to research and provides radiologists and cardiologists with another diagnostic option as they manage their patients with known or suspected CAD."
About Gadavist
Gadavist (gadobutrol) injection was first approved in the U.S. in 2011 for intravenous use in magnetic resonance (MR) imaging in adults and children (2 years of age and older) to detect and visualize areas with disrupted blood brain barrier and/or abnormal vascularity of the central nervous system. Gadavist was further approved in the U.S. in 2014 for MR of the breast in adult patients to assess the presence and extent of malignant breast disease and for pediatric patients less than 2 years of age, including term neonates, to detect and visualize areas with disrupted blood brain barrier and/or abnormal vascularity of the central nervous system. In 2016, it was approved in the U.S. for use with magnetic resonance angiography (MRA) to evaluate known or suspected supra-aortic or renal artery disease in adult and pediatric patients including term neonates.
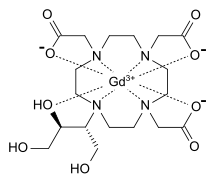
Gadavist, also known as Gadovist® and Gadovist® 1.0 in other regions, is the U.S. brand name of the aqueous 1.0M solution of gadobutrol, a gadolinium (Gd)-based extracellular contrast agent for MRI with a macrocyclic structure. The safety profile of Gadavist has been established in clinical trials involving 7,713 patients (including 184 pediatric patients ages 0-17). The safety and effectiveness of Gadavist have not been established in preterm neonates for any indication or in pediatric patients of any age for use with MR to assess the presence and extent of malignant breast disease, or for use in cardiac MR to assess myocardial perfusion (stress, rest) and late gadolinium enhancement in patients with known or suspected coronary artery disease (CAD). Please see Important Safety Information, including Boxed Warning below.
https://en.wikipedia.org/wiki/Gadobutrol