In continuation of my update on aspirin and its uses..
The study is the first to assess aspirin as a preventive measure for patients who have both diabetes and heart failure. Aspirin, a blood thinner, is strongly recommended for patients who have previously had a heart attack or stroke, but guidelines are unclear regarding its use as a preventive measure for patients who have cardiovascular risk factors but no history of heart attack or stroke. Previous studies in people who have not had those types of health events have shown conflicting evidence of aspirin's potential benefits in the general population. In patients with heart failure, some studies suggest a daily aspirin may even be harmful.
About 27 million people in the U.S. have Type 2 diabetes and about 6.5 million U.S. adults have heart failure, a condition in which the heart becomes too weak to pump enough blood to meet the body's needs. Each condition is associated with an elevated risk of cardiac events, including heart attack and stroke. This study sheds new light on the potential risks and benefits of aspirin for people with both conditions.
"We were surprised to see a paradoxical increase in nonfatal heart attacks and nonfatal stroke, parallel to the decrease in mortality," said Charbel Abi Khalil, MD, PhD, assistant professor of medicine at Weill Cornell Medicine-Qatar and the study's lead author. "This finding might be due to the fact that those patients lived longer; given their mean age of 70 years, perhaps these patients were predisposed to more cardiac events."
Using data from a United Kingdom database known as The Health Improvement Network (THIN), researchers extracted health records of more than 12,000 patients ages 55 and older who had Type 2 diabetes and heart failure but no prior history of heart attack, stroke, peripheral artery disease or atrial fibrillation. Roughly half had been prescribed daily aspirin and half had not.
Researchers analyzed health outcomes over an average of five years of follow-up. All-cause mortality and hospitalization for heart failure were tracked as a composite primary outcome. All-cause mortality, hospitalization for heart failure, major bleeding events and nonfatal heart attack or stroke were tracked separately as secondary outcomes. Those taking a daily aspirin were found to show a 10 percent decrease in the primary outcome, no difference in major bleeding events, and a 50 percent increase in nonfatal heart attack or stroke.
Aspirin interferes with blood's ability to clot, by reducing the activity of platelets, which aggregate during clot formation. Heart failure and diabetes cause changes in the blood that make clot formation more likely, which is why these conditions are associated with a higher risk of heart attacks and strokes.
"Both heart failure and diabetes are associated with increased blood clotting activity," Abi Khalil said. "Because it decreases platelet aggregation, aspirin is thought to lower the likelihood of forming harmful blood clots like those responsible for heart attacks and strokes."
Abi Khalil said patients should speak with their doctors to assess the benefits and risks of taking aspirin.
The research is limited in that it was based on a retrospective analysis of health records, rather than a randomized controlled trial. Further studies would help to confirm the findings, further elucidate the risks and benefits of aspirin use in this patient population, and potentially inform specific guidelines for treatment of patients with diabetes and heart failure.
The study was funded by the biomedical research program at Weill Cornell Medicine-Qatar, a program supported by Qatar Foundation.
Abi Khalil will present the study, "Primary Prevention with Aspirin Reduces Mortality in Type 2 Diabetes and Heart Failure: Results from the THIN Primary Care Database," on Sunday, March 11 at 9:45 a.m. ET in Poster Hall A/B.
The ACC's Annual Scientific Session, which will take place March 10-12 in Orlando, brings together cardiologists and cardiovascular specialists from around the world to share the newest discoveries in treatment and prevention. Follow @ACCinTouch, @ACCMediaCenter and #ACC18 for the latest news from the meeting.

/Soybeans-and-Soy-Milk-5827b45b3df78c6f6a738adf.jpg)

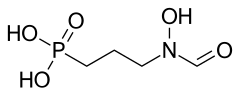 Fosmidomycin
Fosmidomycin  Piperaquine
Piperaquine