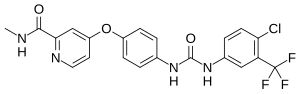
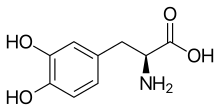
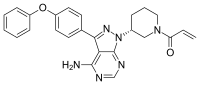
Efforts to treat myotonic dystrophy type 1, the most common form of muscular dystrophy, are in their infancy. In a new study, researchers report they have added new capabilities to an experimental drug agent that previously defeated only one of DM1's many modes of action. Their retooled compounds interrupt the disease's pathology in three ways.
"We've rationally designed something to target multiple pathways, which is contrary to the traditional thinking in medicinal chemistry, where you have one target, one drug," said University of Illinois chemistry professor Steven Zimmerman, who led the research with graduate students Lien Nguyen and Long Luu. "People are slowly discovering that drugs that hit multiple targets are actually better."
The team reports its findings in the Journal of the American Chemical Society.
DM1 (but not Duchennes muscular dystrophy) results from a genetic error that causes expansion of a region of a particular gene, called DMPK. This gene includes a repeated, three-letter sequence of nucleotides, the gene's chemical building blocks. Normal cells contain as many as 35 of these repeats, but sometimes mutation pushes the number of repeats beyond 50, which can lead to symptoms of the disease. Mutant DMPK genes often continue to expand, amplifying the health problems that can result. In some people, the gene includes as many as 10,000 repeats.