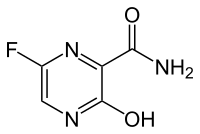
In continuation of my update on Artesunate
Medical experts investigating whether a common malaria drug could have a significant impact on colorectal cancer have launched a crowd funding project to fund their work.
Scientists at St George's, University of London, and St George's Hospital, are in the second phase of research into whether the malaria drug artesunate, can have a positive effect on colorectal cancer patients by reducing the multiplication of tumour cells and decreasing the risk of cancer spreading or recurring after surgery. If it does the drug could be used to provide a cheap adjunct to current expensive chemotherapy.
Artesunate is derived from the plant Artemisia Annua also known as Sweet Wormwood. The Chinese scientist Tu Youyou whose research in the 1960s led to the development of artesunate from a plant used in Chinese traditional medicine, was recently awarded the Nobel Prize 2015.
Over one million patients are diagnosed with colorectal cancer globally each year. Colorectal cancer is the third most common cancer in men and the second most common cancer in women and is a leading cause of mortality. In the UK,110 new cases are diagnosed daily, with older patients particularly at risk of death (Ferlay et al 2014). Current treatments involve complex combinations of surgery, chemotherapy and radiotherapy.
Unfortunately all these measures have not increased overall survival rates beyond 60% at the 5 year stage after patients receive a diagnosis. New treatments are urgently needed to improve survival rates. Developing new, effective drugs however can take many years and sometimes even decades. Repurposing safe and established existing drugs for cancer treatment is therefore gaining interest amongst the scientific community.