Researchers have found that salicylic acid targets the activities of HMGB1, an inflammatory protein associated with a wide variety of diseases, offering hope that more powerful aspirin-like drugs may be developed.
Aspirin is one of the oldest and most commonly used medicines, but many of its beneficial health effects have been hard for scientists and physicians to explain. A recent study conducted by researchers at the Boyce Thompson Institute (BTI), in collaboration with colleagues at Rutgers University and San Raffaele University and Research Institute, shows that aspirin's main breakdown product, salicylic acid, blocks HMGB1, which may explain many of the drug's therapeutic properties. The findings appear Sept. 23, 2015 in the journal Molecular Medicine.
"We've identified what we believe is a key target of aspirin's active form in the body, salicylic acid, which is responsible for some of the many therapeutic effects that aspirin has. This protein, HMGB1, is associated with many prevalent, devastating diseases in humans, including rheumatoid arthritis, heart disease, sepsis and inflammation-associated cancers, such as colorectal cancer and mesothelioma," said senior author Daniel Klessig, a professor at BTI and Cornell University.
Aspirin's pain relieving effects have long been attributed to its ability to block the enzymes cyclooxygenase 1 and 2, which produce prostaglandins--hormone-like compounds that cause inflammation and pain--a discovery that netted its discoverer, John Vane, a Nobel prize. However, the body rapidly converts aspirin to salicylic acid, which is a much less effective inhibitor of cyclooxygenase 1 and 2 than aspirin. Nonetheless, it has similar pharmacological effects as aspirin, suggesting that salicylic acid may interact with additional proteins.
"Some scientists have suggested that salicylic acid should be called 'vitamin S', due to its tremendous beneficial effects on human health, and I concur," said lead author Hyong Woo Choi, a research associate at BTI.
In the current study, researchers discovered the interaction between salicylic acid and HMGB1 by screening extracts prepared from human tissue culture cells to find proteins that could bind to salicylic acid. They identified one of these proteins as HMGB1. These screens have also identified a key suspect in neurodegenerative diseases such as Alzheimer's and Parkinson's diseases, plus approximately two dozen additional candidates that have yet to be characterized.
To further investigate the interactions between salicylic acid and HMGB1's role in the body, Klessig worked with Marco Bianchi of San Raffaele University and Research Institute, who initially discovered that HMGB1 is a trigger of inflammation. Using assays that measured the effects of salicylic acid on the recruitment and activation of immune cells, they showed that salicylic acid could block both of these functions at concentrations similar to those found in people on low-dose aspirin.
"We've found that HMGB1 is involved in countless situations where the body confronts damage to its own cells, which occur in many disease conditions. In retrospect, it's almost obvious that a very general anti-inflammatory compound blocks a very general inflammation trigger," said Bianchi.
Klessig also teamed up with biophysicist Gaetano Montelione at Rutgers, The State University of New Jersey, to not only confirm that salicylic acid can bind to HMGB1, but also to identify the salicylic acid binding sites.
More at research paper




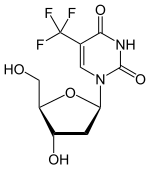 (Trifluridine)
(Trifluridine) 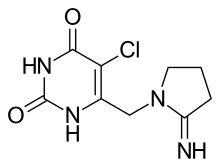 (Tipiracil)
(Tipiracil)
