Cabotegravir long-acting for PrEP is provided as an injection given as few as six times per year and is initiated with a single 600 mg (3-ml) injection given one month apart for two consecutive months. After the second initiation injection, the recommended continuation injection dose is a single 600 mg (3-ml) injection given every two months. Vocabria (cabotegravir oral tablets) may be administered for approximately one month before initiating the first injection to assess the tolerability of the medicine.
Deborah Waterhouse, CEO, ViiV Healthcare, said: “People who are vulnerable to acquiring HIV, especially those in Black and Latinx communities who are disproportionately impacted in the US, may want options beyond daily oral pills. That’s why ViiV Healthcare is proud that Apretude was studied in one of the most diverse and comprehensive HIV prevention trial programs to date, which also included some of the largest numbers of transgender women and Black men who have sex with men ever enrolled in an HIV prevention trial. With Apretude, people can reduce the risk of acquiring HIV with as few as six injections a year. Today’s approval is the latest example of ViiV Healthcare’s commitment to developing long-acting medicines that offer consumers a different choice.”
US FDA approval is based on the results from two international phase IIb/III multicenter, randomised, double-blind, active-controlled trials, HPTN 083 and HPTN 084, which evaluated the safety and efficacy of cabotegravir long-acting for PrEP in HIV-negative men who have sex with men, transgender women, and cisgender women, who were at increased risk of sexually acquiring HIV. In these trials, which included more than 7,700 participants across 13 countries combined, the blinded, randomised portions of both trials were stopped early by an independent Data Safety Monitoring Board after cabotegravir long-acting for PrEP was shown to be superior to daily oral emtricitabine/tenofovir disoproxil fumarate (TDF/FTC) tablets in preventing the acquisition of HIV in study participants. Clinical trial participants who received cabotegravir long-acting for PrEP experienced a 69% lower incidence of HIV compared to FTC/TDF tablets in HPTN 083 and a 90% lower incidence of HIV compared to FTC/TDF tablets in HPTN 084.
The most common adverse reactions (all grades) observed in at least 1% of clinical trial participants receiving cabotegravir long-acting for PrEP were injection site reactions, diarrhoea, headache, pyrexia, fatigue, sleep disorders, nausea, dizziness, flatulence, abdominal pain, vomiting, myalgia, rash, decreased appetite, somnolence, back pain, and upper respiratory tract infection. Adverse events led to discontinuation in 6% of participants in HPTN 083 and 1% of participants in HPTN 084.
In HPTN 083, participants in the US were inclusive of the Black/African American and Latinx communities of men and transgender women who have sex with men, who are disproportionately affected by the HIV epidemic and comprise the greatest percentage of new HIV diagnoses. In HPTN 084, all participants were cisgender women from sub-Saharan Africa. Women in this region bear a disproportionate burden of the HIV epidemic and may be twice as likely to acquire HIV as their male counterparts.
Richard Elion, MD, Director of Research at Washington Health Institute, said: “We have the tools to end the HIV epidemic through the implementation of effective antiretroviral treatment and HIV prevention. PrEP has played a vital role in protecting people from acquiring HIV. With the availability of cabotegravir long-acting for PrEP as an injection every two months to prevent HIV, people now have an important new option besides daily medication. This long-acting medication offers more options for prevention, and now providers and patients will be empowered by choices and the ability to choose the approach that is optimal for each individual.”
HIV continues to be a global public health crisis, with an estimated 38 million people living with HIV worldwide and 1.7 million new cases annually. PrEP represents an effective tool to reduce new cases of HIV, which in addition to successful HIV antiretroviral treatment, will help efforts to end the HIV epidemic. However, fewer than 25% of the people who could benefit from PrEP in the US are currently taking it. Despite the wide availability of daily oral PrEP, it can be limited by inconsistent adherence as well as structural and cultural barriers that lead to underutilisation in key populations.
https://www.drugs.com/apretude.html
https://www.drugs.com/newdrugs/fda-approves-apretude-cabotegravir-extended-release-injectable-suspension-hiv-pre-exposure-5754.html



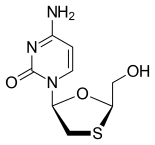

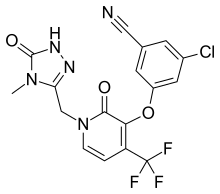
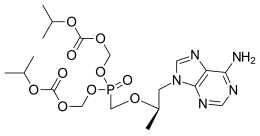

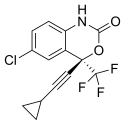

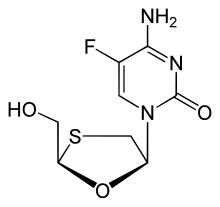 Emtricitabine
Emtricitabine  Rilpivirine
Rilpivirine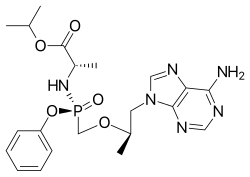 Tenofovir alafenamide
Tenofovir alafenamide

