Rapamycin, an antibiotic and immunosuppressant approved for use about 15 years ago, has drawn extensive interest for its apparent ability at least in laboratory animal tests -- to emulate the ability of dietary restriction in helping animals to live both longer and healthier.
However, this medication has some drawbacks, including an increase in insulin resistance that could set the stage for diabetes. The new findings, published in the Journals of Gerontology: Biological Sciences, help to explain why that happens, and what could be done to address it. They suggest that a combination of rapamycin and another drug to offset that increase in insulin resistance might provide the benefits of this medication without the unwanted side effect.
"This could be an important advance if it helps us find a way to gain the apparent benefits of rapamycin without increasing insulin resistance," said Viviana Perez, an assistant professor in the Department of Biochemistry and Biophysics in the OSU College of Science.
"It could provide a way not only to increase lifespan but to address some age-related diseases and improve general health," Perez said. "We might find a way for people not only to live longer, but to live better and with a higher quality of life."
Age-related diseases include many of the degenerative diseases that affect billions of people around the world and are among the leading causes of death: cardiovascular disease, diabetes, Alzheimer's disease and cancer. Laboratory mice that have received rapamycin have reduced the age-dependent decline in spontaneous activity, demonstrated more fitness, improved cognition and cardiovascular health, had less cancer and lived substantially longer than mice fed a normal diet.
Rapamycin, first discovered from the soils of Easter Island, or Rapa Nui in the South Pacific Ocean, is primarily used as an immunosuppressant to prevent rejection of organs and tissues. In recent years it was also observed that it can function as a metabolic "signaler" that inhibits a biological pathway found in almost all higher life forms -- the ability to sense when food has
been eaten, energy is available and it's okay for cell proliferation, protein synthesis and growth to proceed.
Called mTOR in mammals, for the term "mammalian target of rapamycin," this pathway has a critical evolutionary value -- it helps an organism avoid too much cellular expansion and growth when energy supplies are insufficient. That helps explain why some form of the pathway has been conserved across such a multitude of species, from yeast to fish to humans.
"Dietary restriction is one of the few interventions that inhibits this mTOR pathway," Perez said. "And a restricted diet in laboratory animals has been shown to increase their lifespan about 25-30 percent. Human groups who eat fewer calories, such as some Asian cultures, also live longer."
Aside from a food intake in laboratory mice that's about 40 percent fewer calories than normal, however, it's been found that another way to activate this pathway is with rapamycin, which appears to have a significant impact even when used late in life. Some human clinical trials are already underway exploring this potential.
A big drawback to long-term use of rapamycin, however, is the increase in insulin resistance, observed in both humans and laboratory animals. The new research identified why that is happening. It found that both dietary restriction and rapamycin inhibited lipid synthesis, but only dietary restriction increased the oxidation of those lipids in order to produce energy.
Rapamycin, by contrast, allowed a buildup of fatty acids and eventually an increase in insulin resistance, which in humans can lead to diabetes. However, the drug metformin can address that concern, and is already given to some diabetic patients to increase lipid oxidation. In lab tests, the combined use of rapamycin and metformin prevented the unwanted side effect.
"If proven true, then combined use of metformin and rapamycin for treating aging and age-associated diseases in humans may be possible," the researchers wrote in their conclusion.
This work was supported by the National Institutes of Health. Collaborators included researchers from Oklahoma University Health Science Center, the Oklahoma City VA Medical Center, University of Michigan-Flint, and South Texas Veterans Health Care System.
"There's still substantial work to do, and it may not be realistic to expect with humans what we have been able to accomplish with laboratory animals," Perez said. "People don't live in a cage and eat only the exact diet they are given.
Nonetheless, the potential of this work is exciting."





 rapamycin
rapamycin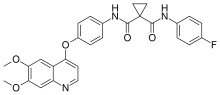
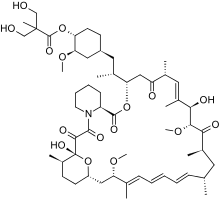 Temsirolimus
Temsirolimus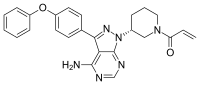 Ibrutinib
Ibrutinib



