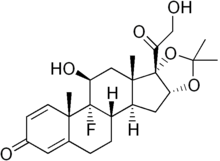
Flexion Therapeutics, Inc. announced, that Food and Drug Administration (FDA) approved Zilretta (triamcinolone acetonide extended-release injectable suspension), the first and only extended-release, intra-articular injection for osteoarthritis knee pain. Zilretta is a non-opioid medicine that employs Flexion's proprietary microsphere technology to provide proven pain relief over 12 weeks.
"The approval of Zilretta marks a major advancement in the treatment landscape for managing OA knee pain," said Michael Clayman, M.D., President and Chief Executive Officer of Flexion. "It comes at a time when our society is in urgent need of non-addictive therapies to help the millions of Americans who suffer from this condition." Dr. Clayman added, "We believe that Zilretta has the potential to be a transformative medicine for the more than five million patients who receive an intra-articular injection for OA knee pain each year."
The FDA approval of Zilretta is based upon data from Flexion's pivotal Phase 3 clinical trial. The randomized, double-blind study enrolled 484 patients at 37 centers worldwide.
Commenting on the approval, Andrew Spitzer, M.D., Co-Director, Joint Replacement Program, Cedars-Sinai Orthopaedic Center, said, "OA knee pain presents a host of challenges for patients and clinicians alike, and there has been very little innovation in this area in recent years. Zilretta is a groundbreaking new therapy, providing clinically meaningful pain relief with a safety profile that is similar to saline."
Sometimes called degenerative joint disease or "wear and tear" arthritis, OA is a progressive and incurable condition and the most common form of arthritis. Its effects may range from intermittent discomfort to the loss of function and severe chronic pain associated with irreversible structural damage.
"As OA progresses, many patients experience intractable joint pain, which can ultimately lead to the need for a total joint replacement," said John Richmond, M.D., Medical Director for Network Development, New England Baptist Hospital. "As a result, healthcare providers are eager for new, non-opioid therapies that may help patients manage their OA pain for extended periods of time. Zilretta gives us an important new non-surgical intervention."
Zilretta's label also includes the results from a double-blind, randomized, parallel-group trial, which examined blood glucose concentrations in patients with type 2 diabetes.
Steven Russell, M.D., Ph.D., Assistant Professor of Medicine, Massachusetts General Hospital Diabetes Research Center, commented, "Our trial demonstrated that Zilretta may avoid the disruptive blood glucose spikes that can be seen with corticosteroid use in patients coping with both knee OA and type 2 diabetes. As a practicing diabetologist, I believe the availability of Zilretta will make intra-articular injection of glucocorticoid an attractive option for these patients."
The pain from OA of the knee can have a profound impact on the people it afflicts, often resulting in a cascade of consequences, which patient advocates warn is only expected to grow.
According to Seth Ginsberg, President and Co-Founder of CreakyJoints®, a national patient advocacy organization for people with all forms of arthritis, "Despite common misperceptions, OA is not a disease that is limited to the elderly. In fact, the average age of knee OA diagnosis has decreased while the number of people diagnosed with OA of the knee has been steadily rising. That's why our community advocates for and welcomes new therapeutic options for people to consider in consultation with their doctor."
Flexion expects Zilretta will be available in the U.S. by the end of October.
Ref : www.Zilretta.com
