A combination of two FDA-approved drugs - a topical chemotherapy and an immune-system-activating compound - was able to rapidly clear actinic keratosis lesions from patients participating in a clinical trial. Standard treatment for this common skin condition, which can lead to the development of squamous cell carcinoma, takes up to a month and can elicit several unpleasant side effects. The report from Massachusetts General Hospital (MGH) investigators has been published online and will appear in the January issue of the Journal of Clinical Investigation.
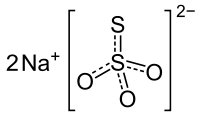
 fluorouracil (5-FU)
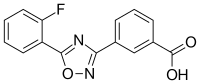
fluorouracil (5-FU)  Calcipotriol
Calcipotriol
"The high tumor clearance rate, short treatment duration and favorable side-effect profile highlight the remarkable effectiveness of this approach, compared with currently available treatments," says Shadmehr Demehri, MD, PhD, of the MGH Center for Cancer Immunology and the Cutaneous Biology Research Center, senior author of the report. "But more importantly, the unprecedented ability of this combination therapy to directly activate the adaptive immune system against skin cancer precursors holds great promise to establish an immune memory within treated skin capable of preventing future cancer development."
Caused by long-term exposure to sunlight, actinic keratosis is characterized by rough, scaly patches on the skin. Very common in older individuals with fair complexions, actinic keratosis is the third most common reason for consulting a dermatologist in the U.S. If untreated, actinic keratosis lesions can progress to squamous cell carcinoma, the second most common form of skin cancer. Current topical treatments for actinic keratosis cause side effects - such as pain, crusting and susceptibility to infection - and need to be applied for up to four weeks.
Calcipotriol, an FDA-approved treatment for psoriasis, induces expression in the skin of an immune system activator called TSLP. While overexpression of TSLP is associated with the allergic inflammation seen in asthma and eczema, it has been noted that individuals with these allergic conditions appear to be less susceptible to skin cancer. Other studies have supported the ability of TSLP to suppress skin cancer development, which led Demehri's team to investigate its potential against actinic keratosis.
Experiments with a mouse model of skin cancer development showed that twice-weekly application of calcipotriol both induced TSLP expression and delayed tumor development. When tumors did develop, they were fewer and smaller than in mice not treated with calcipotriol. An experiment in which calcipotriol was applied to the ears of mice while skin cancer was induced to develop on the backs of the animals resulted in elevated blood levels of TSLP and the suppression of tumor development, implying that brief TSLP-inducing treatment could lead to a lasting systemic antitumor immune response.
Since clinically available concentrations of calcipotriol have had limited effectiveness against actinic keratosis and produced no evidence of immune activation, the MGH team hypothesized that combining the available 0.005 percent calcipotriol ointment with 5 percent fluorouracil (5-FU) cream, a standard treatment for actinic keratosis, might amplify the immune-activating potential of calcipotriol. In a randomized, double-blinded clinical trial, 65 participants with multiple actinic keratosis lesions were treated with a combination of calcipotriol and 5-FU, while 67 received a control preparation of 5-FU mixed with petroleum jelly.
The preparations were applied twice a day to the entire affected sites of participants - face, scalp and arms - and because treatment with 5-FU alone requires seven or more days to have any effect against actinic keratosis, participants were treated for four days only. A day after treatment ended, the treated skin of those receiving calcipotriol plus 5-FU, including areas that did not contain clinically visible lesions, showed clear signs of inflammation, indicating immune system activation. Areas of inflammation were found to have a significant influx of lymphocytes - primarily T cells - at the sites of lesions.
Eight weeks after treatment, participants receiving the combined treatment had a significantly greater reduction in the number and size of actinic keratosis lesions - for example, an average of 88 percent reduction in facial lesions versus 26 percent reduction for those receiving the control preparation. Even participants with large "hypertrophic" lesions, which rarely respond to conventional topical treatments, saw significant reduction in the size of their lesion with combined treatment. Among participants receiving combined treatment who had previously been treated for actinic keratosis, 82 percent found the treatment to be more effective.
"As both medications used in our trial are already available clinically, they could readily be used by dermatologists to treat actinic keratosis, particular in patients for whom conventional treatments have failed," says Demehri, who is an assistant professor of Dermatology at Harvard Medical School. "The ultimate goal of our research is to use patients' own immune systems to prevent cancer, so we are very excited to determine whether our success in activating a T-cell-dominant immune response against a skin cancer precursor will protect the treated skin against future skin cancer development. We're planning to follow the participants in this trial over the coming years to address that important question."


 Trametinib
Trametinib