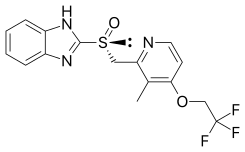
Dexlansoprazole (INN, trade names Kapidex, Dexilant) is a proton pump inhibitor that is marketed by Takeda Pharmaceuticals for the treatment of erosive esophagitis and gastro-oesophageal reflux disease...
Takeda Pharmaceuticals U.S.A., Inc., (Takeda) (TSE: 4502) today announced that the United States (U.S.) Food and Drug Administration (FDA) approved Dexilant SoluTab delayed-release orally disintegrating tablets, a new formulation of dexlansoprazole that can be taken by allowing the tablet to melt in the patient's mouth. Dexilant SoluTab is a proton pump inhibitor (PPI) indicated for the treatment of heartburn associated with symptomatic non-erosive gastroesophageal reflux disease (GERD) and the maintenance of healed erosive esophagitis (EE) and relief of heartburn in adults 18 years and older. Dexilant SoluTab is a PPI with dual delayed release (DDR) technology that is designed to provide two separate releases of medication.
