Teva Pharmaceutical Industries Ltd., announced that the U.S. Food and Drug Administration (FDA) has approved ProAir RespiClick (albuterol sulfate) Inhalation Powder for the treatment or prevention of bronchospasm in children 4 to 11 years of age with reversible obstructive airway disease and for the prevention of exercise-induced bronchospasm (EIB).
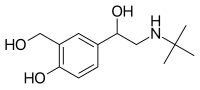
ProAir RespiClick was approved by the FDA for use in patients 12 years of age and older in March 2015 and remains the only breath-activated, multi-dose, dry powder, short-acting beta-agonist (SABA) inhaler available in the U.S.
