Vertex Pharmaceuticals Incorporated announced the U.S. Food and Drug Administration (FDA) approval of Symdeko (tezacaftor/ivacaftor and ivacaftor) for treating the underlying cause of cystic fibrosis (CF) in people ages 12 and older who have two copies of the F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene or who have at least one mutation that is responsive to tezacaftor/ivacaftor. Symdeko is Vertex's third medicine approved to treat the underlying cause of CF. Vertex is ready to launch Symdeko and will begin shipping it to pharmacies in the United States this week.
 Ivacafto
Ivacafto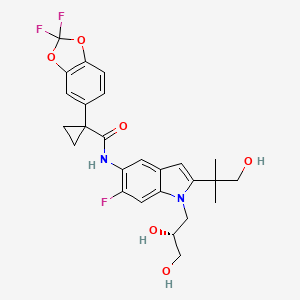 tezacaftor
tezacaftor"Today is an exciting day for the CF community. The approval of Symdeko, our third disease-modifying CF medicine, offers many patients an important new treatment option," said Jeffrey Leiden, M.D., Ph.D., Vertex's Chairman, President and Chief Executive Officer. "This approval is an important milestone in our journey to treat every person with CF, and we remain committed to urgently advancing our efforts to develop new medicines that treat the underlying cause of CF for the many people still waiting."
In November 2017, the New England Journal of Medicine published the results of two Phase 3 studies of Symdeko. These studies, named EVOLVE and EXPAND, enrolled approximately 750 people with CF ages 12 and older with two copies of the F508del mutation or with one F508del mutation and one mutation that results in residual CFTR function. Across both studies, patients treated with Symdeko experienced statistically significant and clinically meaningful improvements in lung function and other measures of disease, with a favorable safety profile. The most common adverse events, regardless of treatment group, included infective pulmonary exacerbation and cough. The first data from the ongoing EXTEND rollover study, also presented in November, show that the lung function improvements and the safety and tolerability profiles seen in EVOLVE and EXPAND were sustained for up to 48 total weeks of Symdeko treatment.
"We've already seen the significant impact that disease-modifying medicines can have on patients and are incredibly pleased that there is now a third treatment option that enables more patients to benefit from CFTR modulation," said Patrick Flume, M.D., Director of the Medical University of South Carolina Cystic Fibrosis Center and Principal Investigator for the EXTEND study. "In particular, Symdeko is an important treatment option for patients who either never started or discontinued Orkambi, and it also provides increased benefit over Kalydeco alone for patients with residual function mutations."
The European Medicines Agency (EMA) has validated the Marketing Authorization Application (MAA) for the tezacaftor/ivacaftor combination. The company expects approval in the EU in the second half of 2018.

No comments:
Post a Comment