Ortho Dermatologics, a division of Valeant Pharmaceuticals International, Inc. (NYSE: VRX and TSX: VRX), today announced that the U.S. Food and Drug Administration (FDA) has accepted the New Drug Application (NDA) for IDP-118 (halobetasol propionate and tazarotene) lotion, an investigational topical treatment for plaque psoriasis. The PDUFA action date is June 18, 2018.
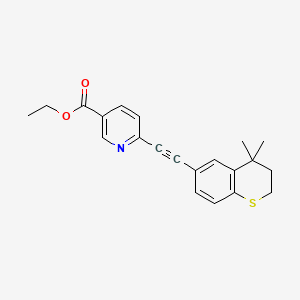 tazarotene
tazarotene 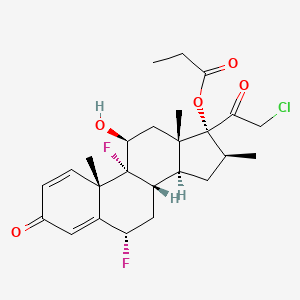
If approved, IDP-118 will be the first and only topical lotion that contains a unique combination of halobetasol propionate and tazarotene in one formulation for the treatment of plaque psoriasis in adult patients, allowing for a potentially expanded duration of use.
The most common adverse events were contact dermatitis (7.4%) and application site pain (2.6%)...

No comments:
Post a Comment